
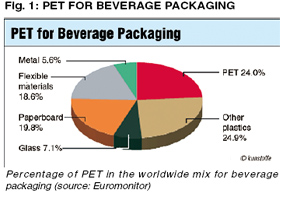
Polyethylene terephthalate (PET) continues to show steady growth. Worldwide consumption is about 45 million tons (source: PCI) and this is expected to grow by about 6% annually in the next few years. PET will thus have reached the highest growth rate of any commodity resin as well as most engineering plastics. Table 1 shows the ten largest PET manufacturers, along with their production capacities. As the largest textile manufacturer, China already has the largest production capacity for polyester resins in the world. Beverage packaging, however, is the primary reason for the high growth rates. With almost 25%, PET represents the largest share of all materials in the worldwide packaging mix (Fig. 1). After a brief spike in September 2006, prices over the last three years have been relatively stable. However, because of the significant increase in the price of oil, rising costs for PET precursors can be expected. This means that a price increase in the PET markets can also be expected. Since, however, not only raw material costs but also transportation costs will increase because of the high price of oil, the weight advantage of PET bottles over glass will become increasingly important. This will likely to further replacement of glass by PET in the beverage area. Raw materials, additives and barrier materials In the packaging sector, PET is hardly found any more as a pure homopolymer. Co-monomers such as isophthalic acid or 1,4-cyclohexane dimethanol are added during polymer izat ion. These comonomers slow crystallization, making faster injection molding cycles possible during perform production. For blow molding bottles, faster cycles are achieved through addition of infrared absorbers to PET raw materials. The performs can then be heated faster. To achieve the low acetaldehyde values required for mineral water applications, either so-called Aqua PET grades with low viscosities are used, or reagents that function as acetaldehyde blockers are added. Lower viscosities result in production of less acetaldehyde during preform production. In contrast, blockers react chemically with acetaldehyde and convert the aldehyde into a poorly diffusing molecule. Anthranilamide is still the most common substance here. Both principles reduce the concentration of acetaldehyde in the bottle wall, so that migration of acetaldehyde into the mineral water is likewise reduced.
"Worldwide consumption of PET reaches 45M tons, with annual growth of 6%." "PET accounts for the biggest share of all materials in the worldwide packaging mix." For oxygen- sensitive beverages such as beer, fruit juices, wine and milk products, the oxygen barrier characteristics of PET alone are not sufficient. The high oxygen permeability can be reduced considerably for these beverages through use of barrier materials. A distinction is drawn here between passive and active barriers or a combination of the two. Polyamide layers placed in the center of multi-layer bottles are often used as passive barriers. The polyamide MXD6 with a cobalt catalyst has been employed since the 1990s as the first active barrier material (CMB Oxbar). Additional materials have appeared since then, for instance, the Aegis The polyamide MXD6 with a cobalt catalyst has been employed since the 1990s as the first active barrier material (CMB Oxbar). Additional materials have appeared since then, for instance, the Aegis product from Honeywell, Heverlee, Belgium, which employs a different polyamide 6 grade as well as an iron-based system from M&G, Tortona, Italy. One problem associated with these active barriers is that the reaction with oxygen begins immediately after production of the preforms. The Polyshield family of products from In-vista, Gersthofen, Germany, which is also based on MXD6 technology, is sold in two components. As a result, the trapping reaction does not be
Womens Running Shoes Polyethylene terephthalate (PET) continues to show steady growth. Worldwide consumption is about 45 million tons (source: PCI) and this is expected to grow by about 6% annually in the next few years. PET will thus have reached the highest growth rate of any commodity resin as well as most engineering plastics. Table 1 shows the ten largest PET manufacturers, along with their production capacities. As the largest textile manufacturer, China already has the largest production capacity for polyester resins in the world. Beverage packaging, however, is the primary reason for the high growth rates. With almost 25%, PET represents the largest share of all materials in the worldwide packaging mix (Fig. 1). After a brief spike in September 2006, prices over the last three years have been relatively stable. However, because of the significant increase in the price of oil, rising costs for PET precursors can be expected. This means that a price increase in the PET markets can also be expected. Since, however, not only raw material costs but also transportation costs will increase because of the high price of oil, the weight advantage of PET bottles over glass will become increasingly important. This will likely to further replacement of glass by PET in the beverage area. Raw materials, additives and barrier materials In the packaging sector, PET is hardly found any more as a pure homopolymer. Co-monomers such as isophthalic acid or 1,4-cyclohexane dimethanol are added during polymer izat ion. These comonomers slow crystallization, making faster injection molding cycles possible during perform production. For blow molding bottles, faster cycles are achieved through addition of infrared absorbers to PET raw materials. The performs can then be heated faster. To achieve the low acetaldehyde values required for mineral water applications, either so-called Aqua PET grades with low viscosities are used, or reagents that function as acetaldehyde blockers are added. Lower viscosities result in production of less acetaldehyde during preform production. In contrast, blockers react chemically with acetaldehyde and convert the aldehyde into a poorly diffusing molecule. Anthranilamide is still the most common substance here. Both principles reduce the concentration of acetaldehyde in the bottle wall, so that migration of acetaldehyde into the mineral water is likewise reduced.
Polyethylene terephthalate (PET) continues to show steady growth. Worldwide consumption is about 45 million tons (source: PCI) and this is expected to grow by about 6% annually in the next few years. PET will thus have reached the highest growth rate of any commodity resin as well as most engineering plastics. Table 1 shows the ten largest PET manufacturers, along with their production capacities. As the largest textile manufacturer, China already has the largest production capacity for polyester resins in the world. Beverage packaging, however, is the primary reason for the high growth rates. With almost 25%, PET represents the largest share of all materials in the worldwide packaging mix (Fig. 1). After a brief spike in September 2006, prices over the last three years have been relatively stable. However, because of the significant increase in the price of oil, rising costs for PET precursors can be expected. This means that a price increase in the PET markets can also be expected. Since, however, not only raw material costs but also transportation costs will increase because of the high price of oil, the weight advantage of PET bottles over glass will become increasingly important. This will likely to further replacement of glass by PET in the beverage area. Raw materials, additives and barrier materials In the packaging sector, PET is hardly found any more as a pure homopolymer. Co-monomers such as isophthalic acid or 1,4-cyclohexane dimethanol are added during polymer izat ion. These comonomers slow crystallization, making faster injection molding cycles possible during perform production. For blow molding bottles, faster cycles are achieved through addition of infrared absorbers to PET raw materials. The performs can then be heated faster. To achieve the low acetaldehyde values required for mineral water applications, either so-called Aqua PET grades with low viscosities are used, or reagents that function as acetaldehyde blockers are added. Lower viscosities result in production of less acetaldehyde during preform production. In contrast, blockers react chemically with acetaldehyde and convert the aldehyde into a poorly diffusing molecule. Anthranilamide is still the most common substance here. Both principles reduce the concentration of acetaldehyde in the bottle wall, so that migration of acetaldehyde into the mineral water is likewise reduced.  "Worldwide consumption of PET reaches 45M tons, with annual growth of 6%." "PET accounts for the biggest share of all materials in the worldwide packaging mix." For oxygen- sensitive beverages such as beer, fruit juices, wine and milk products, the oxygen barrier characteristics of PET alone are not sufficient. The high oxygen permeability can be reduced considerably for these beverages through use of barrier materials. A distinction is drawn here between passive and active barriers or a combination of the two. Polyamide layers placed in the center of multi-layer bottles are often used as passive barriers. The polyamide MXD6 with a cobalt catalyst has been employed since the 1990s as the first active barrier material (CMB Oxbar). Additional materials have appeared since then, for instance, the Aegis The polyamide MXD6 with a cobalt catalyst has been employed since the 1990s as the first active barrier material (CMB Oxbar). Additional materials have appeared since then, for instance, the Aegis product from Honeywell, Heverlee, Belgium, which employs a different polyamide 6 grade as well as an iron-based system from M&G, Tortona, Italy. One problem associated with these active barriers is that the reaction with oxygen begins immediately after production of the preforms. The Polyshield family of products from In-vista, Gersthofen, Germany, which is also based on MXD6 technology, is sold in two components. As a result, the trapping reaction does not beWomens Running Shoes
"Worldwide consumption of PET reaches 45M tons, with annual growth of 6%." "PET accounts for the biggest share of all materials in the worldwide packaging mix." For oxygen- sensitive beverages such as beer, fruit juices, wine and milk products, the oxygen barrier characteristics of PET alone are not sufficient. The high oxygen permeability can be reduced considerably for these beverages through use of barrier materials. A distinction is drawn here between passive and active barriers or a combination of the two. Polyamide layers placed in the center of multi-layer bottles are often used as passive barriers. The polyamide MXD6 with a cobalt catalyst has been employed since the 1990s as the first active barrier material (CMB Oxbar). Additional materials have appeared since then, for instance, the Aegis The polyamide MXD6 with a cobalt catalyst has been employed since the 1990s as the first active barrier material (CMB Oxbar). Additional materials have appeared since then, for instance, the Aegis product from Honeywell, Heverlee, Belgium, which employs a different polyamide 6 grade as well as an iron-based system from M&G, Tortona, Italy. One problem associated with these active barriers is that the reaction with oxygen begins immediately after production of the preforms. The Polyshield family of products from In-vista, Gersthofen, Germany, which is also based on MXD6 technology, is sold in two components. As a result, the trapping reaction does not beWomens Running Shoes













